In today’s world, rapid pathogen detection is critical for preventing outbreaks, protecting crops, and managing global health challenges. While traditional culture-based methods are often too slow and lack sensitivity, advanced pathogen detection systems, especially PCR pathogen detection enable faster, more reliable identification of harmful organisms. These modern solutions are transforming how we respond to emerging threats and ensure safety in healthcare, agriculture, and beyond.
At 3CR Bioscience, we’ve taken PCR-based detection to the next level with our patented PACE® and PACE OneStep RT-PCR technologies. Designed for flexibility and high throughput, our systems are used globally across sectors including agriculture, clinical research, and environmental monitoring. This article explores the benefits of PCR-based pathogen detection systems, with real-world case studies demonstrating the power and versatility of our approach.
What is rapid pathogen detection?
Rapid pathogen detection refers to modern testing methods that quickly identify harmful microorganisms, such as bacteria, viruses, and fungi in a sample. Traditional culture-based techniques can take days to deliver results because pathogens must grow before they can be detected. In contrast, rapid pathogen detection systems use advanced tools like PCR and other molecular assays to detect pathogens directly and much faster.
This speed and accuracy allow healthcare providers, researchers, food producers, and environmental laboratories to act quickly, helping prevent outbreaks, protect public health, and reduce safety risks.
Overview of Modern Pathogen Detection Systems
Modern pathogen detection systems are vital for fast and accurate identification of microorganisms in clinical, agricultural, food, and environmental samples. These rapid pathogen detection technologies improve response times, support public health, and strengthen food and biosafety worldwide.
Typical pathogen detection systems include:
- PCR pathogen detection for sensitive identification of bacteria, viruses, and fungi
- Rapid immunoassays for quick screening in point-of-care settings
- Automated high-throughput platforms for large-scale laboratory testing
- Pathogen detection consumables designed for consistent, reliable workflows
Key benefits of advanced pathogen testing:
- Fast results to prevent outbreaks and guide immediate actions
- High sensitivity and specificity to ensure diagnostic accuracy
- Scalable performance for routine or emergency testing demands
- Versatile application across healthcare, food safety, and environmental monitoring
As demand for rapid and scalable microbiological diagnostics grows, modern systems are transforming global pathogen surveillance and enabling smarter decisions across multiple industries.
PCR Pathogen Detection
PCR pathogen detection is one of the most reliable and widely used methods for identifying harmful microorganisms at the genetic level. By amplifying specific DNA or RNA targets, PCR enables rapid pathogen detection even when only trace amounts of bacteria, viruses, or fungi are present in a sample. This high sensitivity and specificity make PCR the gold standard for pathogen testing in clinical research, agricultural biosecurity, food safety, and environmental monitoring.
Modern PCR-based pathogen detection systems offer:
- Fast turnaround times for timely decision-making
- Accurate detection of both known and emerging pathogens
- Compatibility with high-throughput workflows
- Support for real-time surveillance and outbreak prevention
With advancements such as multiplex assays and automated workflows, PCR pathogen detection continues to evolve, providing faster, scalable solutions that strengthen research for global health and biosafety.
What are pathogen detection consumables?
Pathogen detection consumables are specialized products such as reagents, tubes, PCR plates, pipette tips, and extraction kits used in laboratory testing for the identification of harmful microorganisms. These consumables are designed to ensure the accuracy, reliability, and efficiency of rapid pathogen detection systems by supporting sample preparation, amplification, and analysis processes.
PACE and PACE OneStep RT-PCR: Sensitivity and Precision in High-Throughput PCR Pathogen Detection
At the core of 3CR’s technology is PACE (PCR Allele Competitive Extension)—a patented, high-performance fluorescent PCR genotyping chemistry. PACE enables accurate detection of DNA variants, including single nucleotide polymorphisms (SNPs), across diverse organisms.
Our enhanced PACE OneStep RT-PCR integrates reverse transcription and PCR into a single reaction, allowing for the detection of both DNA- and RNA-based pathogens. This innovation supports rapid pathogen detection workflows that are high-throughput, cost-effective, and easy to adopt.
How PACE Works in Pathogen Detection
PACE assays are tailored to target unique genetic sequences found in pathogen genomes, enabling specific and reliable detection. They can be designed to:
- Identify the presence or absence of a pathogen in a sample.
- Distinguish between strains or variants of the same pathogen.
- Track the evolution of pathogens over time or across populations.
Advantages of Using PACE-Based Systems
Speed: Results in hours, not days.
High Throughput: Ideal for screening hundreds or thousands of samples.
Cost Efficiency: Uses universal reagents with minimal primer requirements.
Flexibility: Detects pathogens in plants, animals, humans, or environmental samples.
Scalability: Easily adapted to different lab workflows or sample volumes.
Case Study: SARS-CoV-2 Variant Detection in Human Populations
During the COVID-19 pandemic, genetic surveillance of viral variants became a global priority. Traditional whole-genome sequencing, though powerful, was expensive and logistically challenging for widespread use.
In response, 3CR Bioscience partnered with the University of Bristol to develop a PACE OneStep RT-PCR pipeline for rapid pathogen detection and variant tracking of SARS-CoV-2. Instead of sequencing entire genomes, the method genotyped SNPs directly from RNA samples to differentiate circulating viral strains.
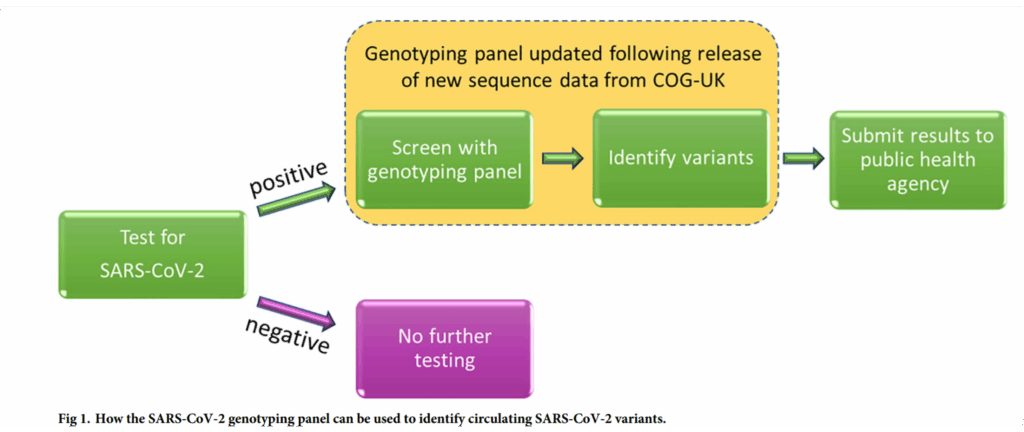
High-Resolution Tracking with Minimal Resources
Using a panel of just 19 SNP markers, the team could distinguish 59 different SARS-CoV-2 genotypes. This rapid, low-cost method provided critical insights into transmission patterns and the emergence of variants, without overburdening testing infrastructure.
The publicly available SNP genotyping pipeline has since been adopted by public health labs for real-time variant monitoring and remains adaptable as new variants arise.
Case Study: Pathogen Detection in Cotton Crops
In agriculture, early pathogen detection is vital to preventing the spread of crop diseases. One such threat is Cotton leafroll dwarf virus (CLRDV)—a single-stranded RNA virus that can cause devastating yield losses.
From Two-Step to One-Step Detection
Researchers at Texas A&M University collaborated with 3CR Bioscience to streamline detection of CLRDV. Building on the lab’s existing use of PACE genotyping, they developed a PACE OneStep RT-PCR assay that combined reverse transcription and DNA amplification into a single step.
The assay accurately differentiated infected and healthy plants and was validated against known sample sets. Its low cost, speed, and simplicity make it ideal for widescale crop monitoring.

Customisable for Other Crop Threats
Thanks to its modular design, PACE and PACE OneStep RT-PCR can be adapted to target other plant pathogens by altering the primer sequences. This makes it a valuable platform for responding to emerging threats in global agriculture.
Pathogen Detection and Beyond
Beyond direct pathogen detection, PACE technology supports broader research in genomics and epidemiology. Its ability to detect SNPs and small insertions/deletions (Indels) enables investigations into:
- Genetic predispositions to human diseases such as cancer and cardiovascular conditions.
- Outbreak investigations involving bacterial, viral, or fungal pathogens.
- Environmental pathogen monitoring in water or soil samples.
- PACE offers a reliable tool for both clinical research and public health surveillance.
Pathogen Testing for Safety and Disease Prevention
Pathogen testing is essential for identifying harmful microorganisms that pose risks to human health, food production, and the environment. By analyzing clinical, agricultural, or industrial samples, pathogen testing ensures early detection of bacteria, viruses, fungi, and other contaminants before they can spread or cause damage. This proactive approach helps prevent disease outbreaks, maintain product quality, and comply with regulatory safety standards.
Pathogen testing is widely used in:
- Healthcare research to guide treatment and protect patients
- Food and beverage safety to prevent contamination and spoilage
- Agriculture and livestock management to detect crop and animal pathogens early
- Environmental monitoring to track water, soil, and air quality
As the need for rapid pathogen detection grows worldwide, advanced molecular methods, including PCR-based diagnostics are replacing slower culture-based testing, enabling faster action and significantly improving biosurveillance and public safety outcomes.
An Open Invitation for Collaboration and Feedback
At 3CR Bioscience, we’re proud to support researchers and organisations working on the front lines of health, agriculture, and environmental safety. We’re continually refining our pathogen detection systems and welcome feedback from our users on how we can improve our solutions or support emerging needs.
We invite you to explore how PACE or PACE OneStep RT-PCR can be adapted for your application. From customised assay development to high-throughput implementation, our team is here to help.
Conclusion
The need for rapid pathogen detection continues to grow across industries. Whether monitoring disease in humans, plants, or animals, fast and accurate screening is essential to mitigating risk, protecting supply chains, and saving lives.
3CR Bioscience’s PCR-based solutions, including PACE and PACE OneStep RT-PCR, provide a scalable, versatile, and cost-effective approach to pathogen detection. Backed by real-world success stories and an ongoing commitment to innovation, our technology is expanding what’s possible redefining the frontiers of molecular diagnostics in agriculture and life sciences.
If you’re looking to implement or improve your pathogen detection system, contact us to learn how PACE can work for you.







